Abstract
STAT3 enhances pro-survival signaling essential for T cell expansion, but when this pathway is not appropriately regulated, aberrant STAT3 signaling contributes to T cell lymphomagenesis.
Cutaneous T cell lymphoma (CTCL) is a type of mature T cell lymphoma characterized by the accumulation of malignant T cells in the skin. Our analysis, along with other recently published sequencing studies, identified upregulation of the STAT3 cytokine signaling pathway as a key driver of CTCL pathogenesis. In normal processes, STAT3 activity is transient, but dysregulation of this pathway is observed in several human malignancies, including CTCL. Aberrant STAT3 activity in malignant cells promotes transcription of target genes that promote proliferation and survival of transformed T cells. Translocations resulting in STAT3 duplications, activating mutations in the SH2 domain of STAT3 and activating mutations in kinases upstream of STAT3 all contribute to hyperactivation of this JAK/STAT signaling pathway in CTCL. STAT3 signaling in bystander cells within the tumor microenvironment may further contribute to tumor progression, although the precise role of this signaling pathway in malignant disease is context dependent.
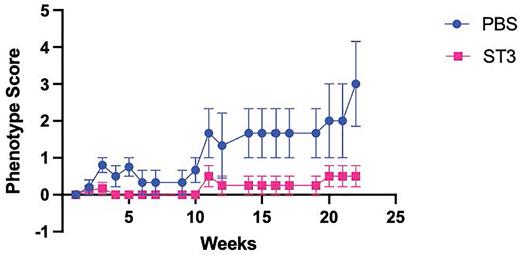
We have used conditional gene targeting to develop a fully penetrant STAT3-dependent small animal model of CTCL that recapitulates many key features of human disease. In this model, a constitutively active form of STAT3 is expressed exclusively in T lymphocytes. The autochthonous mouse model is characterized by progressive accumulation of malignant T cells in the skin and secondary lymphoid organs. Our molecular analysis of T cells from this mouse model revealed a transcriptional signature that largely mirrored the one found in malignant cells from patients. We have now taken advantage of this tractable animal model of CTCL to evaluate the therapeutic potential of a potent and selective E3 ubiquitin ligase-based novel STAT3 heterobifunctional degrader for targeting this difficult-to-treat hematologic malignancy. Weekly intravenous administration of the degrader was well tolerated and led to significant reduction of STAT3 levels in circulating and skin-resident T lymphocytes. Progression of disease in our preclinical model was notably blunted upon administration of the STAT3 degrader with dramatic reduction in T cell accumulation in the skin and lymph nodes. We will report on pre-clinical evaluation of the degrader and the impact on disease progression, T cell accumulation, activation and proliferation in vivo following weekly drug administration. Targeted protein degradation represents a novel therapeutic modality enabling direct targeting of previously undruggable oncoproteins such as STAT3. These data provide a rationale for selective STAT3 degradation as a therapeutic strategy for malignancies such as CTCL that are associated with constitutive activation of STAT3 signaling.
Fig Legend:Treatment of mice exhibiting CTCL phenotypes starting at ~3mo of age. Phenotype score reflects presentation of fur loss, scaling/flaking of the skin, appearance of lesions and death.
Disclosures
Dey:KymeraTx: Current Employment. Yang:KymeraTx: Current Employment. Gollerkeri:KymeraTx: Current Employment. Gollob:KymeraTx: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal